Silver Ions vs. Nanoparticles for Antibacterial Wound Dressings
For centuries, silver has served as an anti-microbial agent, effectively targeting bacteria, fungi and algae. Ancient civilizations like the Greeks and Romans harnessed the medicinal properties of silver by applying silver-based ointments or silver-coated dressings for infection control and wound healing. The effectiveness of silver ions derived from dissolved silver nitrate, silver sulphate and silver sulfadiazine against both Gram-positive and Gram-negative bacteria has already been demonstrated. Along with ionic silver, silver nanoparticles have also gained attraction as antimicrobial agents with the dynamic development of nanotechnology. This article further compares the use of ionic silver and silver nanoparticles for antimicrobial wound dressings.
Silver ions vs. Silver Nanoparticles:
Ionic silver refers to silver in its ionic form which carries a positive charge (Ag+). Nano-silver, on the other hand, consists of extremely small silver particles, typically ranging in size from 1 to 100 nm and having a neutral electric charge. In aqueous solutions, silver can be ionized to form silver ions. These ions are highly reactive and are commonly used in various applications such as wound dressings, water purification systems, medical devices, etc. due to their ability to inhibit the growth of bacteria, fungi and other microorganisms. Silver nanoparticles can be synthesized into a variety of shapes and sizes (Figure 1), by various methods such as chemical reduction, green synthesis, laser ablation, electron/gamma irradiation, chemical or photochemical synthesis or biological synthetic processes.
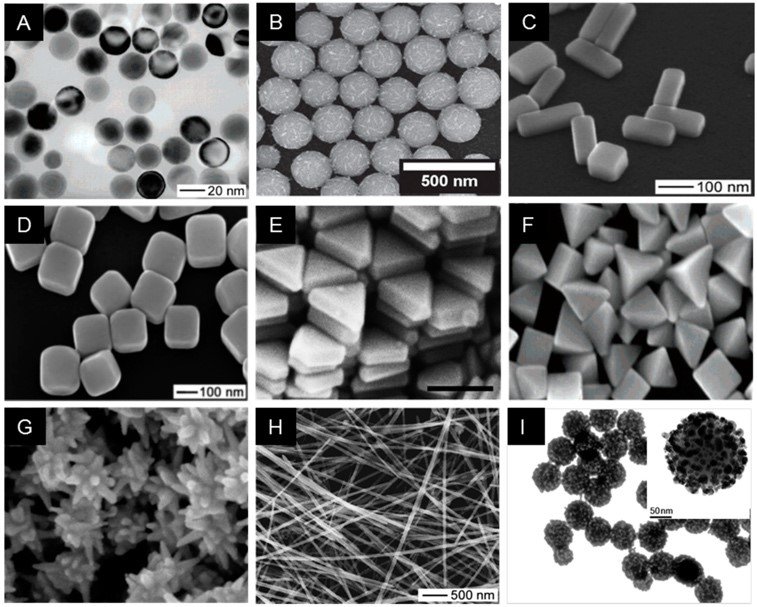
Figure 1: Depicts the different shapes and sizes of silver nanoparticles. Image source – [2]
How do Silver Ions kill Bacteria?
The antibacterial activity of silver ions can be attributed to multiple pathways (Figure 2). Positively charged silver ions are adsorbed on the cell membrane by binding to bacterial proteins. The binding affects the integrity of the membrane leading to membrane damage and disturbed membrane permeability. (a) Silver ions are also known to inactivate proteins and interfere with the normal metabolism of bacteria. (b) Silver ions show bactericidal activity by interacting with respiratory enzymes like dehydrogenases, thereby inhibiting the respiratory chain (c and e). Silver ions can also bind to DNA bases and form cross-links, resulting in DNA shrinkage, destruction of DNA structure and inhibition of DNA replication, causing cell death (d). Disturbances in the respiratory chain can also lead to uncontrolled production of ROS (reactive oxygen species), further aggravating the DNA and membrane damage. Silver ions are also known to inhibit ROS-scavenging enzymes, resulting in the induction of oxidative stress (e).
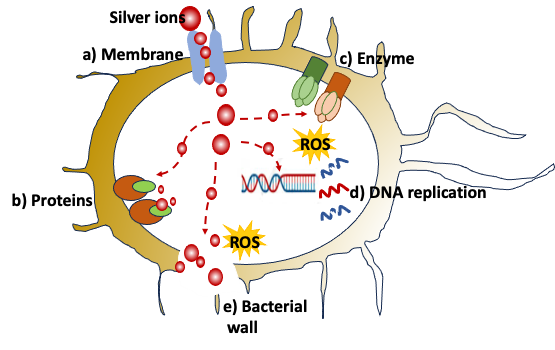
Figure 2: Antibacterial action of silver. Image source – [1]
Silver Wound Dressings – Which Ones Are More Effective?
Silver-based wound dressings utilize the antimicrobial properties of either ionic silver or silver nanoparticles for the management of wound infections. A variety of ionic silver impregnated dressings are available in the market in various forms such as foams, gels, films, etc. Common examples include silver sulfadiazine or silver alginate dressings. Another type, Silver releasing dressings, allow sustained and controlled release of silver ions over time. Sustained release ensures prolonged antimicrobial activity and is helpful in the management of chronic or slow-healing wounds.
Apart from ionic silver, silver nanoparticles are also explored for their antimicrobial potential. Dressings with silver nanoparticles offer effective antimicrobial activity due to their high surface area to volume ratio. Though silver nanoparticles are found effective as antimicrobial agents, ionic silver is preferred over silver nanoparticles because silver nanoparticles have a high cost of production, variable effectiveness, stability issues, etc. Upon long-term storage, silver nanoparticles form aggregates and undergo oxidation, resulting in accumulation effect at the site – leading to toxicity. Furthermore, silver nanoparticles can easily penetrate the skin barrier and may enter the bloodstream. They may damage mitochondria and may enter the embryo and circulation system. On the other hand, ionic silver is stable, has a low production cost, and provides gradual release from wound dressings, providing sustained antimicrobial activity.
Serigen’s Seriderm-Ag silver ion wound dressing incorporates the power of ionic silver to combat wound infections. Seriderm-Ag allows a sustained release of silver ions over the period of 7 days, which helps in infection control while reducing the need for frequent dressing changes. Seriderm-Ag exhibits broad-spectrum antimicrobial activity against a wide range of microorganisms, including multi-drug resistant organisms, thereby being suitable for a diverse range of infected or high-risk wounds.
Conclusion
Silver ions are preferred over silver nanoparticles for preventing and controlling wound infections. Seriderm-Ag has a double advantage – the healing power of silk and the antimicrobial action of ionic silver for infected or high-risk wounds such as diabetic foot ulcers, pressure ulcers and second-degree burns. Therefore, using our silver ion wound dressing for these wounds can support faster healing and efficient infection control.
References
- Zhiwen Xu, Cai Zhang, Xiang Wang, and Dingbin Liu. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 5, 3985–3999
- Sang Hun Lee and Bong-Hyun Jun. Silver Nanoparticles: Synthesis and Application for Nanomedicine. J. Mol. Sci. 2019, 20, 865
- Wilkinson, R. J. White, J. K. Chipman. Silver and nanoparticles of silver in wound dressings: A review of efficacy and safety. Journal of wound care, 2011, 20(11)

Author Bio –
Dr. Rucha Deshpande is Manager R&D at Serigen Mediproducts. She holds a PhD in Zoology from the University of Pune and has 7 years of professional experience in tissue engineering, cell culture and molecular biology.
.

For centuries, silver has served as an anti-microbial agent, effectively targeting bacteria, fungi and algae. Ancient civilizations like the Greeks and Romans harnessed the medicinal properties of silver by applying silver-based ointments or silver-coated dressings for infection control and wound healing. The effectiveness of silver ions derived from dissolved silver nitrate, silver sulphate and silver sulfadiazine against both Gram-positive and Gram-negative bacteria has already been demonstrated. Along with ionic silver, silver nanoparticles have also gained attraction as antimicrobial agents with the dynamic development of nanotechnology. This article further compares the use of ionic silver and silver nanoparticles for antimicrobial wound dressings.
Silver ions vs. Silver Nanoparticles:
Ionic silver refers to silver in its ionic form which carries a positive charge (Ag+). Nano-silver, on the other hand, consists of extremely small silver particles, typically ranging in size from 1 to 100 nm and having a neutral electric charge. In aqueous solutions, silver can be ionized to form silver ions. These ions are highly reactive and are commonly used in various applications such as wound dressings, water purification systems, medical devices, etc. due to their ability to inhibit the growth of bacteria, fungi and other microorganisms. Silver nanoparticles can be synthesized into a variety of shapes and sizes (Figure 1), by various methods such as chemical reduction, green synthesis, laser ablation, electron/gamma irradiation, chemical or photochemical synthesis or biological synthetic processes.

Figure 1: Depicts the different shapes and sizes of silver nanoparticles. Image source – [2]
How do Silver Ions kill Bacteria?
The antibacterial activity of silver ions can be attributed to multiple pathways (Figure 2). Positively charged silver ions are adsorbed on the cell membrane by binding to bacterial proteins. The binding affects the integrity of the membrane leading to membrane damage and disturbed membrane permeability. (a) Silver ions are also known to inactivate proteins and interfere with the normal metabolism of bacteria. (b) Silver ions show bactericidal activity by interacting with respiratory enzymes like dehydrogenases, thereby inhibiting the respiratory chain (c and e). Silver ions can also bind to DNA bases and form cross-links, resulting in DNA shrinkage, destruction of DNA structure and inhibition of DNA replication, causing cell death (d). Disturbances in the respiratory chain can also lead to uncontrolled production of ROS (reactive oxygen species), further aggravating the DNA and membrane damage. Silver ions are also known to inhibit ROS-scavenging enzymes, resulting in the induction of oxidative stress (e).

Figure 2: Antibacterial action of silver. Image source – [1]
Silver Wound Dressings – Which Ones Are More Effective?
Silver-based wound dressings utilize the antimicrobial properties of either ionic silver or silver nanoparticles for the management of wound infections. A variety of ionic silver impregnated dressings are available in the market in various forms such as foams, gels, films, etc. Common examples include silver sulfadiazine or silver alginate dressings. Another type, Silver releasing dressings, allow sustained and controlled release of silver ions over time. Sustained release ensures prolonged antimicrobial activity and is helpful in the management of chronic or slow-healing wounds.
Apart from ionic silver, silver nanoparticles are also explored for their antimicrobial potential. Dressings with silver nanoparticles offer effective antimicrobial activity due to their high surface area to volume ratio. Though silver nanoparticles are found effective as antimicrobial agents, ionic silver is preferred over silver nanoparticles because silver nanoparticles have a high cost of production, variable effectiveness, stability issues, etc. Upon long-term storage, silver nanoparticles form aggregates and undergo oxidation, resulting in accumulation effect at the site – leading to toxicity. Furthermore, silver nanoparticles can easily penetrate the skin barrier and may enter the bloodstream. They may damage mitochondria and may enter the embryo and circulation system. On the other hand, ionic silver is stable, has a low production cost, and provides gradual release from wound dressings, providing sustained antimicrobial activity.
Serigen’s Seriderm-Ag silver ion wound dressing incorporates the power of ionic silver to combat wound infections. Seriderm-Ag allows a sustained release of silver ions over the period of 7 days, which helps in infection control while reducing the need for frequent dressing changes. Seriderm-Ag exhibits broad-spectrum antimicrobial activity against a wide range of microorganisms, including multi-drug resistant organisms, thereby being suitable for a diverse range of infected or high-risk wounds.
Conclusion
Silver ions are preferred over silver nanoparticles for preventing and controlling wound infections. Seriderm-Ag has a double advantage – the healing power of silk and the antimicrobial action of ionic silver for infected or high-risk wounds such as diabetic foot ulcers, pressure ulcers and second-degree burns. Therefore, using our silver ion wound dressing for these wounds can support faster healing and efficient infection control.
References
- Zhiwen Xu, Cai Zhang, Xiang Wang, and Dingbin Liu. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 5, 3985–3999
- Sang Hun Lee and Bong-Hyun Jun. Silver Nanoparticles: Synthesis and Application for Nanomedicine. J. Mol. Sci. 2019, 20, 865
- Wilkinson, R. J. White, J. K. Chipman. Silver and nanoparticles of silver in wound dressings: A review of efficacy and safety. Journal of wound care, 2011, 20(11)

Author Bio –
Dr. Rucha Deshpande is Manager R&D at Serigen Mediproducts. She holds a PhD in Zoology from the University of Pune and has 7 years of professional experience in tissue engineering, cell culture and molecular biology.